Muscimol

| |
| |

| |
| Names | |
|---|---|
| IUPAC name
5-(Aminomethyl)-1,2-oxazol-3(2H)-one
| |
| Other names
Agarin, Pantherine, Agarine, Pantherin
| |
| Identifiers | |
3D model (JSmol)
|
|
| 774694 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.574 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| UN number | 2811 3077 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties[1] | |
| C4H6N2O2 | |
| Molar mass | 114.104 g·mol−1 |
| Melting point | 184 to 185 °C (363 to 365 °F; 457 to 458 K) |
| very soluble | |
| Solubility in ethanol | slightly soluble |
| Solubility in methanol | very soluble |
| Pharmacology | |
| Legal status |
|
| Hazards | |
| GHS labelling:[2] | |
| H300 | |
| P264, P270, P301+P316, P321, P330, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Muscimol (also known as agarin or pantherine) is one of the principal psychoactive constituents of Amanita muscaria and related species of mushroom. Muscimol is a potent and selective orthosteric agonist for the GABAA receptor[3] and displays sedative-hypnotic, depressant and hallucinogenic[citation needed] psychoactivity. This colorless or white solid is classified as an isoxazole.
Muscimol underwent a phase I clinical trial for epilepsy, but the trial was discontinued.[4]
Muscimol, an agonist for the GABAA receptor, was able to significantly alleviate pain in its peak effect, recent studies from 2023 show. It has since been federally banned in Australia and is pending FDA review in the United States, but scientists believe it may relieve pain as well as some opioids without much of the risk of addiction associated with opioids. [5]
Biochemistry
[edit]The main natural sources of muscimol are fungi of the genus Amanita, such as Amanita muscaria (fly agaric) and Amanita pantherina (panther amanita). It is produced in the mushrooms along with muscarine (which is present in trace amounts and it is not active), muscazone, and ibotenic acid.[6][7] In A. muscaria, the layer just below the skin of the cap contains the highest amount of muscimol, and is therefore the most psychoactive portion.[8]
Muscimol is recognized as a potent agonist for ionotropic GABA-A receptors. By mimicking the inhibitory neurotransmitter GABA, muscimol activates these receptors, leading to the opening of chloride channels and subsequent hyperpolarization of neurons. This results in decreased neuronal excitability, which is crucial for maintaining the balance between excitation and inhibition in the central nervous system.[9]
The biochemical properties of muscimol make it a valuable tool for investigating GABAergic mechanisms. Its high affinity and specificity for GABA-A receptors allow researchers to study synaptic transmission, neural circuit dynamics, and the overall role of GABAergic inhibition in various physiological and pathological states.[9]

Pharmacology
[edit]
Muscimol is a potent GABAA agonist, activating the receptor for the brain's principal inhibitory neurotransmitter, GABA. Muscimol binds to the same site on the GABAA receptor complex as GABA itself, as opposed to other GABAergic drugs such as barbiturates and benzodiazepines which bind to separate regulatory sites.[10] GABAA receptors are widely distributed in the brain, and so when muscimol is administered, it alters neuronal activity in multiple regions including the cerebral cortex, hippocampus, and cerebellum. While muscimol is normally thought of as a selective GABAA agonist with exceptionally high affinity to GABAA-delta receptors,[11][12][13] it is also a partial agonist at the GABAA-rho receptor, and so its range of effects results from a combined action on more than one GABAA receptor subtype.[14]
Scientific studies have shown that dosing of the active ingredient muscimol is usually not precise as it has to be extracted from dried amanita mushroom. However, a psychoactive dose of muscimol is reported to be between 8 and 15 mg. As little as a gram of dried Amanita muscaria button may contain this amount of muscimol; however, the potency varies greatly among mushrooms.[15]
When consumed, a substantial percentage of muscimol goes un-metabolized and thus excreted in urine, a phenomenon exploited by Siberian practitioners of the traditional entheogenic use of Amanita muscaria.[16]
In patients with Huntington's disease and chronic schizophrenia, oral doses of muscimol have been found to cause a rise of both prolactin and growth hormone.[17]
During a test involving rabbits connected to an EEG, muscimol presented with a distinctly synchronized EEG tracing. This is substantially different from serotonergic psychedelics, with which brainwave patterns generally show a desynchronization. In higher doses (2 mg/kg via IV), the EEG will show characteristic spikes.[18]
Mechanism of action
[edit]Muscimol primarily functions as a GABA-A receptor agonist, mimicking the action of GABA, the main inhibitory neurotransmitter, in the central nervous system.
Effects
[edit]Muscimol, as a GABA-A receptor agonist, has shown diverse pharmacological effects, spanning neuroprotective, anti-nociceptive, and anti-epileptic activity.[19]
Recent research has highlighted the following effects of muscimol:
- Neurotransmission Modulation: By mimicking GABA and binding to GABA-A receptors, muscimol enhances inhibitory neurotransmission. This results in reduced neuronal firing rates, contributing to the overall calming effect on the CNS. This modulation is crucial in maintaining the balance between excitatory and inhibitory signals in the brain.[20]
- Migraine and Headache: Studies on migraine models demonstrated that extrasynaptic GABA-A receptor agonists like muscimol could prevent migraine-like phenotypes, offering new avenues for migraine treatment.[21]
- Depressant Effects: By enhancing inhibitory neurotransmission, muscimol acts as a CNS depressant. This can lead to muscle relaxation, reduction in anxiety.[22]
- Antinociceptive Properties: Muscimol has been found to have antinociceptive effects when used in combination with citalopram, a selective serotonin reuptake inhibitor. This additive effect highlights muscimol's potential in pain management.[23]
- Decision Making and Cognitive Function: Research on the role of the rat prelimbic cortex indicated that muscimol can influence decision-making processes. By infusing muscimol, researchers observed significant changes in cortical activity, which are crucial for understanding cognitive functions and cognitive disorders.[24]
- Cerebral Ischemic Injury: Muscimol's role in alleviating cerebral ischemic injury was explored, revealing its ability to suppress oxidative stress, autophagy, and apoptosis pathways. This research underscores muscimol's potential in treating ischemic conditions.[25]
- Pain Management: Activation of 5-HT5A receptors in the ventrolateral orbital cortex, alongside GABA-A receptor modulation by muscimol, showed significant antinociceptive effects in models of neuropathic pain and inflammatory pain.[26]
- Epilepsy Models: In studies involving absence epilepsy models, muscimol demonstrated effects on T-type calcium channels and GABA receptors, providing insights into its anticonvulsant properties.[27]
- Substance Use Disorders: Research into sex differences in GABA receptor regulation highlighted muscimol's potential in addressing cocaine use disorder, emphasizing its role in GABAergic modulation.[citation needed]
- Neurological Pathways: Investigations into neural pathways for internal bias and sensory information interaction in decision-making processes showed the significant impact of muscimol on visual cortex neurons.[citation needed]
Clinical research
[edit]Muscimol underwent a phase I clinical trial for epilepsy in 2012, but this trial was discontinued.[4]
A handful of exploratory clinical trials (n≤10) probing the utility of muscimol in the treatment of schizophrenia, Huntington's disease, and tardive dyskinesia occurred between 1977-1982.[28][29][30] The clinical investigation of muscimol in these conditions was superseded by gaboxadol, a conformationally constrained derivative of muscimol.[31] Neither drug appeared to be efficacious in the treatment of Huntington's disease or L-dopa/neuroleptic induced dyskinesia.[32][33]
Muscimol-based products
[edit]Muscimol, a psychoactive compound derived from the ibotenic acid found in certain mushrooms, particularly Amanita muscaria, has garnered significant interest due to its unique effects on the nervous system. Muscimol binds to GABA receptors in the brain, resulting in its sedative and hallucinogenic properties.[citation needed] Muscimol-based products are currently being investigated for their potential therapeutic applications, especially in the treatment of anxiety, insomnia, and other neurological disorders.[34][35] The psychoactive nature of muscimol necessitates stringent regulation and cautious usage to ensure safety.[36] However, ongoing research aims to harness its medicinal benefits in a controlled context, highlighting the broader scientific interest in natural compounds as potential sources for novel medical treatments.[citation needed]
Chemistry
[edit]Structure
[edit]Muscimol was first isolated from Amanita pantherina by Onda in 1964,[37] and thought to be an amino acid or peptide. Structure was then elucidated by Takemoto,[38] Eugster,[39] and Bowden.[40] Muscimol is a semi-rigid isoxazole containing both alcohol and aminomethyl substituents.[41] Muscimol is commonly portrayed as a tautomer, where it adopts an amide-like configuration.[2] It is also commonly shown as a zwitterion.[42]
Isolation
[edit]Muscimol can be extracted from the flesh of the Amanita muscaria by treatment with boiling water, followed by rapid cooling, and further treatment with a basic resin. This is washed with water, and eluted with acetic acid using column chromatography. The eluate is freeze dried, dissolved in water, and passed down a column of cellulose phosphate.[43] A subsequent elution with ammonium hydroxide and recrystallization from alcohol results in pure muscimol.[44]
In instances where pure muscimol is not required, such as recreational or spiritual use, a crude extract is often prepared by simmering dried Amanita muscaria in water for thirty minutes.[45]
Chemical synthesis
[edit]Muscimol was synthesized in 1965 by Gagneux,[46] who utilized a bromo-isoxazole starting material in a two step reaction. 3-bromo-5-aminomethyl-isoxazole (1) was refluxed in a mixture of methanol and potassium hydroxide for 30 hours, resulting in 3-methoxy-5-aminomethyl-isoxazole (2) with a yield of 60%.

(2) was then refluxed in concentrated hydrochloric acid to hydrolyze the methoxy group, and the zwitterion crystallized from a solution of methanol and tetrahydrofuran after the addition of triethylamine, resulting in a 50% yield.[46]

Chemists report having struggled to reproduce these results.[47][48] More dependable and scalable procedures have been developed, two examples being the syntheses of McCarry[49] and Varasi.[42]
McCarry's synthesis is a three step synthesis involving a lithium acetylide produced from propargyl chloride. The acetylide (3), was dissolved in ether, cooled to -40 °C, and treated with excess ethyl chloroformate to afford ethyl 4-chlorotetrolate (4) in a 70% yield. (4) was then added to a solution of water, methanol and hydroxylamine at -35 °C. At a pH of between 8.5 and 9, the isoxazole (5) was recovered in a 41% yield. Muscimol was formed in a 65% yield when (5) was dissolved in a saturated solution of methanol and anhydrous ammonia and heated from 0 °C to 50 °C. The total yield was 18.7%.[49]

Varasi's synthesis is notable for its inexpensive starting materials and mild conditions. It begins with the combination of 2,3-Dichloro-1-propene (6), potassium bicarbonate, water, and dibromoformaldoxime (7) (which is a well known precursor of bromo nitriloxyde, a reactive dipole for regioselective Diels-Alder cycloadditions, which forms in alkali), all dissolved in ethyl acetate. 5-Chloromethyl-3-bromoisoxazole (8) was extracted with an experimental yield of 81%. 5-Aminomethyl-3-bromoisoxazole (9) was formed in 90% yield by the combination of (8) and ammonium hydroxide in dioxane.[42]
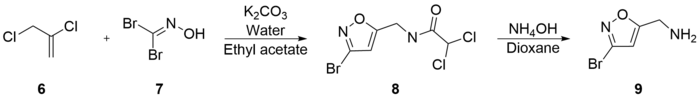
(9) was then refluxed with potassium hydroxide in methanol to generate 5-Aminomethyl-3-methoxyisoxazole (10) with a 66% yield. Subsequent reflux of (10) with hydrobromic acid and acetic acid generated muscimol with a yield of 62%. The overall synthetic yield was 30%.[42]

Toxicity
[edit]The toxicity and safety profile of Muscimol have been studied in various contexts, both experimental and clinical.
Dose-Dependent Effects in Primates
[edit]A study on nonhuman primates indicated that muscimol, when administered in escalating doses, caused reversible hyperkinesia and dyskinesias at higher doses (up to 88.8 mM), but no long-term toxicity was observed on histological examination.[50]
The median lethal dose in mice is 3.8 mg/kg s.c, 2.5 mg/kg i.p. The LD50 in rats is 4.5 mg/kg i.v, 45 mg/kg orally.[51]
Anticonvulsant Properties
[edit]Muscimol has shown potential as an anticonvulsant, blocking seizures induced by various agents in animal models without causing significant toxicity at therapeutic doses.[52]
Human Poisoning Cases
[edit]A retrospective review of muscimol poisoning cases from Amanita mushrooms indicated that symptoms included gastrointestinal upset, CNS excitation, but no deaths were reported. Most symptoms resolved within 24 hours.[53]
Distribution and Metabolism
[edit]Studies on muscimol's distribution in rats showed it enters the brain and is metabolized rapidly, suggesting that its toxicity is low when used in controlled doses.[citation needed]
Muscimol exhibits dose-dependent effects with higher doses leading to significant, but reversible, CNS symptoms.[54] Its toxicity appears to be low when used in controlled environments, with no long-term damage observed in animal studies and human cases resolving without severe outcomes. However, caution is advised with its use due to its potent effects on the central nervous system.[citation needed]
Legal status
[edit]Australia
[edit]Muscimol is considered a Schedule 9 prohibited substance in Australia under the Poisons Standard (October 2015). A Schedule 9 substance is a substance "which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities."[55]
United States
[edit]Neither Amanita muscaria nor muscimol is considered a controlled substance by the Federal government of the United States. This means that cultivation, possession, and distribution are unregulated by the United States Federal Government.[56][57] The legality of Amanita muscaria and muscimol as ingredients in food is unclear since neither are approved as food additives by the FDA. However, agriculture regulators in Florida actioned against one seller of Amanita products after the agency had determined such products were considered adulterated under state law.[58]
Muscimol may be regulated on a state level. Louisiana State Act 159 banned the possession and cultivation of the Amanita muscaria except for ornamental or aesthetic purposes. Except as a constituent of lawfully manufactured food or dietary supplements, the act outlaws preparations of the Amanita muscaria intended for human consumption, including muscimol.[59]
See also
[edit]References
[edit]- ^ "Muscimol". The Merck Index Online.
- ^ a b "Muscimol". PubChem.
- ^ Johnston GA (October 2014). "Muscimol as an ionotropic GABA receptor agonist". Neurochemical Research. 39 (10): 1942–1947. doi:10.1007/s11064-014-1245-y. PMID 24473816. S2CID 13364321.
- ^ a b Heiss JD, Walbridge S, Rene'Smith RN, Sato S, Oldfield EH, Lonser RR (August 2012). "174 Convection-Enhanced Delivery of Muscimol to the Epileptic FocusPreclinical and Clinical Research". Neurosurgery. 71 (2): E568. doi:10.1227/01.neu.0000417764.02569.dc. ISSN 0148-396X.
- ^ Ramawad HA, Paridari P, Jabermoradi S, Gharin P, Toloui A, Safari S, Yousefifard M (2023). "Muscimol as a treatment for nerve injury-related neuropathic pain: A systematic review and meta-analysis of preclinical studies". The Korean Journal of Pain. 36 (4): 425–440. doi:10.3344/kjp.23161. PMC 10551397. PMID 37732408.
- ^ Chilton WS, Ott J (1976). "Toxic metabolites of Amanita pantherina, A. cothurnata, A. muscaria and other Amanita species". Lloydia. 39 (2–3): 150–157. PMID 985999.
- ^ a b Michelot D, Melendez-Howell LM (February 2003). "Amanita muscaria: chemistry, biology, toxicology, and ethnomycology". Mycological Research. 107 (Pt 2): 131–146. doi:10.1017/S0953756203007305. PMID 12747324.
- ^ Chilton WS (1978). "Chemistry and Mode of Action of Mushroom Toxins". In Rumack BH, Salzman E (eds.). Mushroom Poisoning: Diagnosis and Treatment. CRC Press. pp. 87–124. ISBN 978-0-8493-5185-3.
- ^ a b "The Psychopharmacology of Muscimol: From Mushrooms to Neuroscience". Technology Networks. Retrieved 2024-06-19.[dead link]
- ^ Frølund B, Ebert B, Kristiansen U, Liljefors T, Krogsgaard-Larsen P (August 2002). "GABA(A) receptor ligands and their therapeutic potentials". Current Topics in Medicinal Chemistry. 2 (8): 817–832. doi:10.2174/1568026023393525. PMID 12171573.
- ^ Quirk K, Whiting PJ, Ragan CI, McKernan RM (August 1995). "Characterisation of delta-subunit containing GABAA receptors from rat brain". European Journal of Pharmacology. 290 (3): 175–181. doi:10.1016/0922-4106(95)00061-5. PMID 7589211.
- ^ Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, et al. (October 2006). "GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol". Proceedings of the National Academy of Sciences of the United States of America. 103 (41): 15230–15235. Bibcode:2006PNAS..10315230C. doi:10.1073/pnas.0604304103. PMC 1578762. PMID 17005728.
- ^ Benkherouf AY, Taina KR, Meera P, Aalto AJ, Li XG, Soini SL, et al. (April 2019). "Extrasynaptic δ-GABAA receptors are high-affinity muscimol receptors". Journal of Neurochemistry. 149 (1): 41–53. doi:10.1111/jnc.14646. PMC 6438731. PMID 30565258.
- ^ Woodward RM, Polenzani L, Miledi R (April 1993). "Characterization of bicuculline/baclofen-insensitive (rho-like) gamma-aminobutyric acid receptors expressed in Xenopus oocytes. II. Pharmacology of gamma-aminobutyric acidA and gamma-aminobutyric acidB receptor agonists and antagonists". Molecular Pharmacology. 43 (4): 609–625. PMID 8386310.
- ^ Peredy T, Bruce R (2014). "Mushrooms, Ibotenic Acid". Encyclopedia of Toxicology. pp. 412–413. doi:10.1016/B978-0-12-386454-3.00756-9. ISBN 978-0-12-386455-0.
- ^ "Another Magic Mushroom" p. 228 in: Goldstein A (2001). "Hallucinogens". Addiction. pp. 219–232. doi:10.1093/oso/9780195146639.003.0014. ISBN 978-0-19-514663-9.
- ^ Tamminga CA, Neophytides A, Chase TN, Frohman LA (December 1978). "Stimulation of prolactin and growth hormone secretion by muscimol, a gamma-aminobutyric acid agonist". The Journal of Clinical Endocrinology and Metabolism. 47 (6): 1348–1351. doi:10.1210/jcem-47-6-1348. PMID 162520.
- ^ De Carolis AS, Lipparini F, Longo VG (January 1969). "Neuropharmacological investigations on muscimol, a psychotropic drug extracted from Amanita muscaria". Psychopharmacologia. 15 (3): 186–195. doi:10.1007/BF00411168. PMID 5389124. S2CID 26824149.
- ^ Kaur S, Singh S, Arora A, Ram P, Kumar S, Kumar P, Abed SN (2020). "Pharmacology of GABA and Its Receptors". Frontiers in Pharmacology of Neurotransmitters. pp. 241–292. doi:10.1007/978-981-15-3556-7_8. ISBN 978-981-15-3555-0.
- ^ Chandra D, Halonen LM, Linden AM, Procaccini C, Hellsten K, Homanics GE, Korpi ER (March 2010). "Prototypic GABAA Receptor Agonist Muscimol Acts Preferentially Through Forebrain High-Affinity Binding Sites". Neuropsychopharmacology. 35 (4): 999–1007. doi:10.1038/npp.2009.203. PMC 2823376. PMID 20032968.
- ^ Alpay B, Cimen B, Akaydin E, Onat F, Bolay H, Sara Y (10 May 2024). "Extrasynaptic δGABAA receptors mediate resistance to migraine-like phenotype in rats". The Journal of Headache and Pain. 25 (1): 75. doi:10.1186/s10194-024-01777-4. PMC 11083752. PMID 38724972.
- ^ Petrack B, Yokoyama N (1985). "Anti-Anxiety Agents and Sedative-Hypnotics". Annual Reports in Medicinal Chemistry. Vol. 20. pp. 1–9. doi:10.1016/S0065-7743(08)61027-1. ISBN 978-0-12-040520-6.
- ^ Shokrnejad-namin T, Amini E, Khakpai F, Zarrindast MR (December 2024). "The additive effect between citalopram and muscimol upon induction of antinociceptive effect in male mice". IBRO Neuroscience Reports. 17: 58–64. doi:10.1016/j.ibneur.2024.05.003.
- ^ Palmer JA, White SR, Lopez KC, Laubach M (19 March 2024). "The role of rat prelimbic cortex in decision making". bioRxiv: 2024.03.18.585593. doi:10.1101/2024.03.18.585593. PMC 10983993. PMID 38562679.
- ^ Lan J, Wang J, Wang S, Wang J, Huang S, Wang Y, Ma Y (3 February 2024). "The Activation of GABAAR Alleviated Cerebral Ischemic Injury via the Suppression of Oxidative Stress, Autophagy, and Apoptosis Pathways". Antioxidants. 13 (2): 194. doi:10.3390/antiox13020194. PMC 10886019. PMID 38397792. Gale A784035674.
- ^ Zhao YL, Xu JL, Yi HY, Baba SS, Guo YX, Hou XM, Yuan XC, Li XH, Wang YY, Liang LL, Huo FQ (March 2024). "Activation of 5-HT5A receptor in the ventrolateral orbital cortex produces antinociceptive effects in rat models of neuropathic and inflammatory pain". Neuropharmacology. 245: 109830. doi:10.1016/j.neuropharm.2023.109830. PMID 38160874.
- ^ Tiryaki ES, Arslan G, Günaydın C, Ayyıldız M, Ağar E (March 2024). "The role of HCN channels on the effects of T-type calcium channels and GABAA receptors in the absence epilepsy model of WAG/Rij rats". Pflügers Archiv - European Journal of Physiology. 476 (3): 337–350. doi:10.1007/s00424-023-02900-1. PMID 38159130.
- ^ Tamminga CA, Crayton JW, Chase TN (June 1978). "Muscimol: GABA agonist therapy in schizophrenia". The American Journal of Psychiatry. 135 (6): 746–747. doi:10.1176/ajp.135.6.746. ISSN 0002-953X. PMID 350058.
- ^ Cassady SL, Thaker GK, Moran M, Birt A, Tamminga CA (1992-08-15). "GABA agonist-induced changes in motor, oculomotor, and attention measures correlate in schizophrenics with tardive dyskinesia". Biological Psychiatry. 32 (4): 302–311. doi:10.1016/0006-3223(92)90035-x. ISSN 0006-3223. PMID 1358231.
- ^ Shoulson I, Goldblatt D, Charlton M, Joynt RJ (1977). "Huntington's disease: treatment with muscimol, a GABA-mimetic drug". Transactions of the American Neurological Association. 102: 124–125. ISSN 0065-9479. PMID 150672.
- ^ Foster NL, Chase TN, Denaro A, Hare TA, Tamminga CA (May 1983). "THIP treatment of Huntington's disease". Neurology. 33 (5): 637–639. doi:10.1212/wnl.33.5.637. ISSN 0028-3878. PMID 6221200.
- ^ Foster NL, Chase TN, Denaro A, Hare TA, Tamminga CA (May 1983). "THIP treatment of Huntington's disease". Neurology. 33 (5): 637–639. doi:10.1212/wnl.33.5.637. ISSN 0028-3878. PMID 6221200.
- ^ Morselli PL, Fournier V, Bossi L, Musch B (1985). "Clinical Activity of GABA Agonists in Neuroleptic- and L-Dopa-Induced Dyskinesia". Dyskinesia. Psychopharmacology Supplementum. Vol. 2. pp. 128–136. doi:10.1007/978-3-642-70140-5_17. ISBN 978-3-642-70142-9. ISSN 0179-8456. PMID 2860656.
{{cite book}}:|journal=ignored (help) - ^ Lyvers M, Meester M (November 2012). "Illicit Use of LSD or Psilocybin, but not MDMA or Nonpsychedelic Drugs, is Associated with Mystical Experiences in a Dose-Dependent Manner" (PDF). Journal of Psychoactive Drugs. 44 (5): 410–417. doi:10.1080/02791072.2012.736842. PMID 23457892.
- ^ Abramsohn Y, Peles E, Potik D, Schreiber S, Adelson M (September 2009). "Sense of Coherence as a Stable Predictor for Methadone Maintenance Treatment (MMT) Outcome". Journal of Psychoactive Drugs. 41 (3): 249–253. doi:10.1080/02791072.2009.10400535. PMID 19999678.
- ^ Halpern J (March 2003). "Hallucinogen persisting perception disorder: what do we know after 50 years?". Drug and Alcohol Dependence. 69 (2): 109–119. doi:10.1016/S0376-8716(02)00306-X. PMID 12609692.
- ^ Onda M, Fukushima H, Akagawa M (June 1964). "A Flycidal Constituent of Amanita pantherina (DC.) FR". Chemical & Pharmaceutical Bulletin. 12 (6): 751. doi:10.1248/cpb.12.751. PMID 14199180.
- ^ イボテン酸の構造 [Structure of Ibotenic Acid] (in Japanese). pp. 1232–1233. in: Takemoto T, Nakajima T, Yokobe T, Sakuma R, Fujitani K, Aoyagi Y, Masaki Y (1964). "寄書" [Communication to the Editor]. Yakugaku Zasshi (in Japanese). 84 (12): 1230–1236. doi:10.1248/yakushi1947.84.12_1230.
- ^ Eugster C, Müller G, Good R (January 1965). "Wirkstoffe aus amanita muscaria: ibotensaeure und muscazon" [The active ingredients from Amanita muscaria: ibotenic acid and muscazone]. Tetrahedron Letters (in German). 6 (23): 1813–1815. doi:10.1016/s0040-4039(00)90133-3. PMID 5891631.
- ^ Bowden K, Drysdale AC, Mogey GA (June 1965). "Constituents of Amanita muscaria". Nature. 206 (991): 1359–1360. Bibcode:1965Natur.206.1359B. doi:10.1038/2061359a0. PMID 5891274. S2CID 4178793.
- ^ Brehm L, Frydenvang K, Hansen LM, Norrby PO, Krogsgaard-Larsen P, Liljefors T (December 1997). "Structural features of muscimol, a potent GABAA receptor agonist, crystal structure and quantum chemicalab initio calculations". Structural Chemistry. 8 (6): 443–451. doi:10.1007/BF02311703. S2CID 93397543.
- ^ a b c d "An Improved Synthesis of Muscimol". Synthetic Communications. 22 (13): 1939–1948. July 1992. doi:10.1080/00397919208021324.
- ^ "Cellulose Phosphate: Product Information" (PDF). Sigma Aldrich. Retrieved 23 April 2020.
- ^ Bowden K, Drysdale A (1965). "A novel constituent of Amanita muscaria". Tetrahedron Letters. 6 (12): 727–728. doi:10.1016/s0040-4039(01)83973-3. PMID 14291871.
- ^ Heinrich C (23 November 2008). "Amanita muscaria Preparation for Beginners". Erowid.[self-published source?]
- ^ a b Gagneux AR, Häfliger F, Eugster CH, Good R (January 1965). "Synthesis of pantherine (agarin)". Tetrahedron Letters. 6 (25): 2077–2079. doi:10.1016/S0040-4039(00)90157-6.
- ^ Chiarino D, Napoletano M, Sala A (1986). "A convenient synthesis of muscimol by a 1,3-dipolar cycloaddition reaction". Tetrahedron Letters. 27 (27): 3181–3182. doi:10.1016/S0040-4039(00)84748-6.
- ^ Bowden K, Crank G, Ross WJ (1968). "The synthesis of pantherine and related compounds". Journal of the Chemical Society C: Organic: 172. doi:10.1039/j39680000172.
- ^ a b McCarry BE, Savard M (January 1981). "A facile synthesis of muscimol". Tetrahedron Letters. 22 (51): 5153–5156. doi:10.1016/S0040-4039(01)92445-1.
- ^ Heiss JD, Walbridge S, Asthagiri AR, Lonser RR (April 2010). "Image-guided convection-enhanced delivery of muscimol to the primate brain: Laboratory investigation". Journal of Neurosurgery. 112 (4): 790–795. doi:10.3171/2009.7.JNS09652. PMC 2853729. PMID 19715424.
- ^ "Psychoactive Amanitas Chemistry". Erowid.[unreliable source?]
- ^ Collins RC (June 1980). "Anticonvulsant effects of muscimol". Neurology. 30 (6): 575–581. doi:10.1212/wnl.30.6.575. PMID 7189834.
- ^ Moss MJ, Hendrickson RG (February 2019). "Toxicity of muscimol and ibotenic acid containing mushrooms reported to a regional poison control center from 2002–2016". Clinical Toxicology. 57 (2): 99–103. doi:10.1080/15563650.2018.1497169. PMID 30073844.
- ^ Farber NB, Jiang X, Dikranian K, Nemmers B (December 2003). "Muscimol prevents NMDA antagonist neurotoxicity by activating GABAA receptors in several brain regions". Brain Research. 993 (1–2): 90–100. doi:10.1016/j.brainres.2003.09.002. PMID 14642834.
- ^ "Poisons Standard". The Government of Australia. October 2015.
- ^ "Controlled Substance Schedules". deadiversion.usdoj.gov. US Department of Justice. Archived from the original on 21 November 2020. Retrieved 6 May 2020.
- ^ "Psychoactive Amanitas: Legal Status". Erowid.[unreliable source?]
- ^ Sam O (May 31, 2023). "Mood-altering mushroom sales bloom despite safety concerns". Health News Florida. Retrieved June 6, 2023.
- ^ "Louisiana Act No 159". Louisiana State Legislature. Archived from the original on 25 January 2024. Retrieved 6 May 2020.
